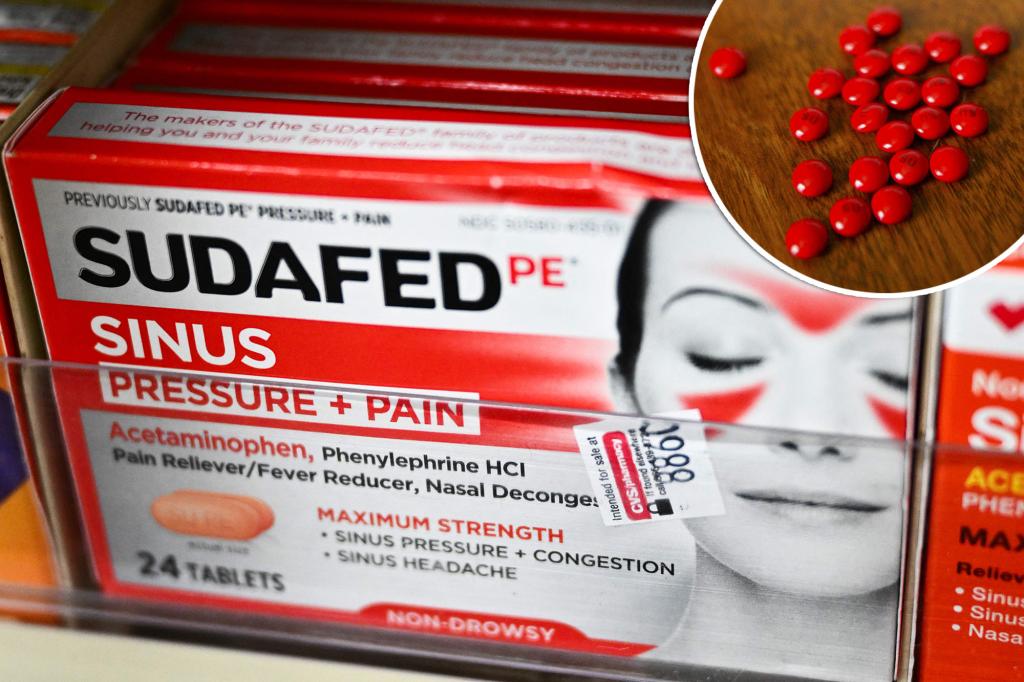
The Food and Drug Administration (FDA) is fighting Sudafed and other decongestants to the streets.
The agency announced Thursday that it will allow the public to comment on recent findings that phenylephrine, the active ingredient in dozens of oral decongestants, is ineffective.
On Tuesday of this week, an FDA advisory panel announced – after examining decades of documentation and clinical trial results – that oral phenylephrine is no better than placebo in reducing nasal congestion.
The advisory panel’s surprising report is not binding, although the FDA generally sides with the panel’s opinion when determining the safety or effectiveness of a drug.
Nasal sprays containing phenylephrine are not affected by the regulation and will remain available for purchase over the counter (OTC).
“Many OTC drugs, including phenylephrine, are sold because they contain ingredients generally recognized by the FDA as safe and effective (GRASE) when used as recommended,” the agency said in a news release.
If the FDA sides with their advisory panel, then the agency will issue a proposed order to remove all oral medications containing phenylephrine from store shelves.
 The FDA will soon allow public comment on a proposal to remove popular decongestants like Sudafed from store shelves.Getty Images
The FDA will soon allow public comment on a proposal to remove popular decongestants like Sudafed from store shelves.Getty Images
“The public will then have the opportunity to comment on the proposed order. If, after considering the comments, FDA continues to conclude phenylephrine is ineffective, the agency will issue a final order … and phenylephrine will no longer be considered GRASE,” the FDA announced.
The FDA, founded more than 100 years ago with the passage of the Pure Food and Drug Act of 1906, must by law allow public comment on its regulations.
“FDA routinely allows ample time for public input (typically 60 days) and considers these comments carefully when it drafts a final rule,” the agency said on its website. The public may submit comments about any proposed rule directly to the agency by mail or online at www.regulations.gov.
Phenylephrine is found in many well-known brand-name OTC oral preparations, including:
- Sudafed PE
- Alka-Seltzer Plus Cold & Flu Formula
- Mucinex
- Robitussin Peak Cold Nighttime Nasal Relief
- Synex
- Dayquil
- Benadryl
- Sinus Tylenol
- Advil sinus congestion
- Lusonal
- Many store brands such as Equate, Walgreens, CVS, etc.
 Phenylephrine is found in many well-known, brand-name OTC oral decongestants. AP
Phenylephrine is found in many well-known, brand-name OTC oral decongestants. AP
Phenylephrine came to prominence in 2006 after another decongestant, pseudoephedrine, was pulled from drugstore shelves because it was an ingredient in the illegal stimulant methamphetamine.
After the passage of the Combat Methamphetamine Epidemic Act of 2005, pseudoephedrine was only available over the counter, so drugmakers replaced it with phenylephrine-based OTC products.
However, when an FDA panel analyzed early studies used to support the use of OTC phenylephrine, they found that study results were inconsistent, did not meet modern standards for study design or had flawed data.
“[W]I believe that the original study was methodologically unsound and does not match today’s standards,” said Dr. Peter Starke, the FDA official who led the recent phenylephrine study.
“Instead, we believe the new data are reliable and do not provide evidence that oral phenylephrine is effective as a nasal decongestant,” Starke added.
The market for OTC decongestants is huge: A consumer survey of 100,000 US households showed that about half buy drugs with phenylephrine throughout the year, and most of them do so several times a year.
Categories: Trending
Source: thtrangdai.edu.vn/en/