Eye drops sold at Walmart are being added to the Food and Drug Administration’s (FDA) list of potentially dangerous products.
Consumers are now being told to avoid Equate Hydration PF Lubricant Eye Drop 10 mL, which has been removed from Walmart store shelves and its online marketplace, due to the potential risk of eye infection that could cause partial or complete vision loss, according to the latest notice from the FDA.
Last week, federal health regulators first warned consumers to either avoid or throw away 26 other products intended to treat dry or irritated eyes after an investigation revealed that there were “unsanitary conditions” at the manufacturing facility, potentially contaminating the products. intended. be sterile.
There were also positive bacterial test results from environmental sampling of critical drug production areas at the facility, the FDA said.
The FDA is warning people not to buy or use certain eye drops from some major brands because of the risk of eye infections. @FDA_Drug_Info / X
The agency did not disclose what bacteria was found and did not link this warning to a previous outbreak of antibiotic-resistant pseudomonas aeruginosa bacteria linked to eye products from Global Pharma Healthcare.
Walmart said in a statement that it takes seriously “quality and safety standards for all of our suppliers” and that it is working with manufacturers.
In addition to removing items from stores, Walmart also implemented sales blocks at its registers to prevent any future purchases.
Buyers are advised to stay away from Equate Hydration PF Lubricant Eye Drop 10 mL. Getty Images/iStockphoto
The 26 products previously listed are marketed under the CVS Health, Leader (Cardinal Health), Rugby (Cardinal Health), Rite Aid, Velocity Pharma and Target’s Up & Up brands.
CVS, Rite Aid and Target were already in the process of removing products from their store shelves and online marketplaces when the warning was posted last week.
However, the regulator warned that “products branded as Leader, Rugby and Velocity may still be available for purchase in stores and online.”
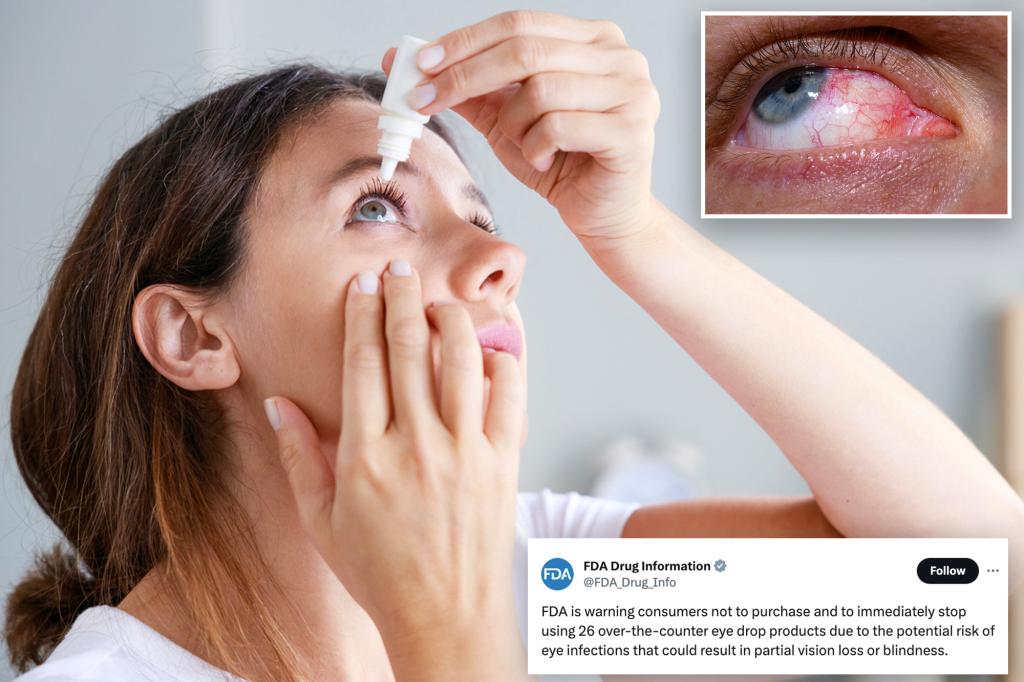
The FDA is warning consumers not to buy and immediately stop using 26 over-the-counter eye drop products because of the potential risk of eye infection that can result in partial vision loss or blindness.
For more info & product list: https://t.co/ngXBwLNOAC pic.twitter.com/T92SH0fMca
— FDA Drug Info (@FDA_Drug_Info) October 30, 2023
The FDA did not specify the manufacturer, but several companies stated that Velocity Pharma supplied the product.
There have been no reports of eye infections associated with this product to date.
However, users who have signs or symptoms of an eye infection after using this product are told to seek medical attention “immediately.”
Certain eye drops, which cause eye infections, can cause partial or complete vision loss, according to a recent notice from the FDA. Getty Images
Symptoms to look out for
Symptoms of an eye infection include irritated or red eyes; worsening pain in or around the eye – even after contact lens removal; light sensitivity; sudden blurred vision; or watery eyes or unusual discharge, according to the Centers for Disease Control and Prevention.
Patients are encouraged to report any adverse events or quality problems they experience with any medication to the agency’s MedWatch Adverse Event Reporting program.
Meanwhile, CVS, which immediately withdrew products supplied by Velocity Pharma in the CVS Health Brand Eye Products portfolio after being notified by the FDA, is offering consumers refunds for the products.
CVS interesting products supplied by Velocity Pharma in the CVS Health Brand Eye Product portfolio after being notified by the FDA. Getty Images/iStockphoto
“We are committed to ensuring that the products we offer are safe, perform as expected and satisfy customers, and are cooperating fully with the FDA on this matter,” a CVS spokesperson told FOX Business.
Cardinal Health told FOX Business that it is placing all identified affected eye drop products in its inventory, and is working with Velocity Pharma and the FDA to initiate a recall of all affected Rugby Lab and Cardinal Health Leader branded eye drop products.
“We are working with Velocity Pharma, the supplier of the affected eye drop products, to gain additional insight into the unsanitary conditions identified by the FDA at the manufacturing facility,” Cardinal Health said in a statement.
Target, Rite Aid and Velocity Pharma did not immediately respond to FOX Business’ requests for comment.
Categories: Trending
Source: thtrangdai.edu.vn/en/